The Food Industry’s False Claims About Front-of-Package Nutrition Labeling: A Fact Sheet
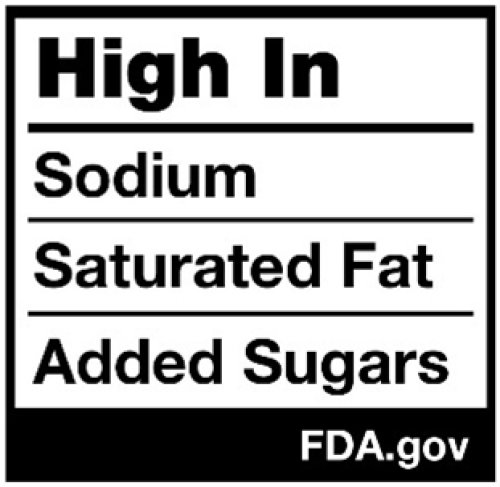
The U.S. Food and Drug Administration (FDA) is developing a policy to require consumer-friendly, front-of-package nutrition labels on foods and beverages sold in the U.S. The agency may well adopt labels to help consumers compare the healthfulness of different foods at a glance by clearly identifying products that are high in added sugars, sodium, and saturated fats. Food industry lobbyists make cynical claims to oppose mandatory front-of-package labeling, but the facts do not support these claims.
Claim: Changing labels presents a high cost to industry, which will be passed on to consumers.
Facts:
- Front-of-package labeling did not increase food prices in Chile. In the 18 months following the implementation of the country’s front-of-package labeling law, prices across foods with and without “High In” labels remained unchanged relative to pre-law trends.
- Companies change their labels frequently and the new labels can be part of periodic redesigns. If the food industry can change labels for holidays and movie premieres, why not for public health and transparency?
- Labeling costs are a drop in the bucket for companies that spend billions on advertising each year. Last time FDA required updates to nutrition labeling, the agency estimated they would cost industry less than $3,500 per product in 2014 dollars, or about $4,500 in 2024 dollars. In contrast, Kraft Heinz spent about $750 million on advertising per quarter in 2023.
- Exemptions and longer compliance periods will minimize the cost burden for small businesses. Whenever it introduces new food labeling requirements, FDA gives smaller companies extra time to comply. For example, FDA gave manufacturers with less than $10 million in annual food sales an extra year to implement updates to the Nutrition Facts label. Sellers with less than $500,000 in annual gross sales to consumers are completely exempt from nutrition labeling requirements and are likely to be exempt from front-of-package labeling as well.

Claim: Front-of-package labeling will effectively characterize foods as “good” or “bad” based on just three nutrients.
Facts:
- FDA’s front-of-package labels will not judge foods as good or bad, nor will they tell people which foods they should or should not eat. They will simply provide information by displaying objective characterizations of the levels of specific nutrients identified as nutrients to limit in the Dietary Guidelines for Americans. These will help people understand the nutritional content of individual products in the context of their overall daily diet. Nutrition Facts panels and ingredients lists will remain on packages to provide more detail.
Claim: Front-of-package labels focused only on nutrients to limit, and not positive nutrients or food groups, are not aligned with the Dietary Guidelines for Americans.
Facts:
- The Dietary Guidelines emphasize the importance of following a healthy dietary pattern consisting of nutrient-dense forms of foods and beverages across all food groups, defined as forms with the least amounts of added sugars, saturated fats, and sodium. Highlighting high levels of these nutrients is directly aligned with the goal of enabling consumers to quickly and easily identify foods that can help them build healthy eating patterns.

- FDA has a complementary initiative to develop an "FDA Healthy” symbol for foods. This icon will be allowed on products that provide servings of healthy food groups and can be used in conjunction with front-of-package labeling to facilitate healthier choices.
- Companies already highlight the presence of positive nutrients and healthy food groups on labels, and requiring nutrients to limit will ensure more accurate representation of products’ full nutritional profiles. A study conducted by the FDA from 2006-2007 found that more than half of foods in U.S. grocery stores had nutrient claims like “High Fiber” and “Low Sodium.” A quick walk through the grocery store reveals that these claims remain common today. Front-of-package labeling will help address misleading marketing, or “health-washing,” of foods containing certain positive attributes but also nutrients we should limit in our diets.

Claim: The industry’s voluntary front-of-package labeling initiative, Facts Up Front, provides sufficient information to guide consumers toward healthier choices.
Facts:

- Not a single study has found that Facts Up Front-style labels encourage healthier choices. The system was first created in 2011. If it were effective, the food industry would have produced the evidence by now.
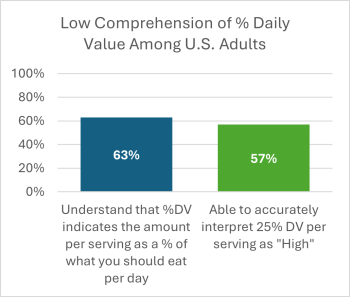
- Facts Up Front relies on the percent Daily Value, which isn’t well understood by consumers. In an FDA survey from 2019, only 63 percent of respondents could accurately interpret the %DV, including less than half of respondents without a high school degree or GED. Effective front-of-package labels would not just present the %DV (which is already on the Nutrition Facts label), but make it easier to interpret.
Claim: There is not enough evidence that front-of-package labeling will lead to meaningful behavioral changes and public health gains.
Facts:
- Extensive scientific evidence demonstrates that front-of-package labels highlighting high levels of sugar, sodium, and saturated fat can improve consumer understanding, encourage healthier diets, and even improve the quality of the national food supply. In contrast, there is no evidence that the industry’s voluntary Facts Up Front system affects consumer choices or spurs reformulation. Fifteen countries have already decided there is sufficient data and passed mandatory front-of-package labeling policies to protect their citizens. The U.S. can’t sit by and wait for decades-long follow-up studies from other countries examining long-term health outcomes while our own chronic disease epidemics rage on. We must act now!
For more information, please contact the Center for Science in the Public Interest: policy@cspinet.org.
This factsheet has been paid for by CSPI Action Fund.
Tags
Topics

