Walking the immunity tightrope

Beyond the curve: Dr. Peter Lurie's COVID-19 blog
Pop Quiz: What does the dietary supplement industry have in common with Zoom, Clorox, and Nintendo?
Answer: All of their sales are booming during the COVID-19 pandemic.
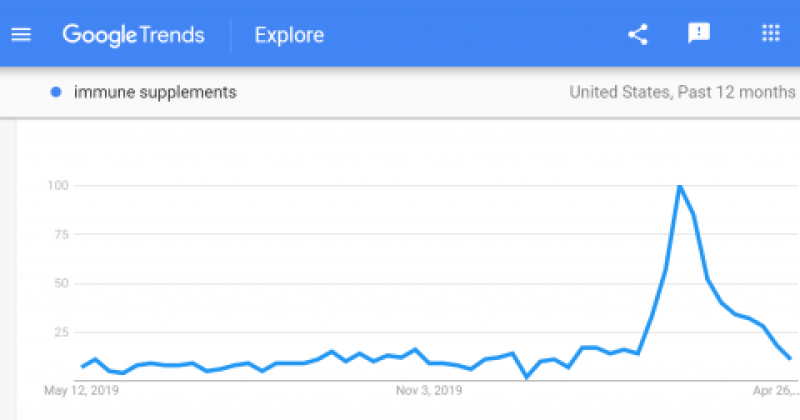
In the eight weeks ending April 18, 2020, vitamin sales were up nearly 50% above sales at the same time last year. And Google Trends shows a massive spike in searches for “immune supplements” since February. My post from May 14 discussed why claims that foods and supplements can boost immunity and protect against the coronavirus are generally bogus. So why are these companies allowed to scam the public, and what is being done about it?
Dietary supplements are among the most poorly regulated consumer products sold in the U.S. Unlike drugs and food additives, most supplements undergo little or no premarket review for either safety or effectiveness. You can hang this debacle on the 1994 Dietary Supplement Health and Education Act (DSHEA), which established a bare-bones system of oversight by the Food and Drug Administration (FDA). But the law has limited ability to protect consumers from misleading claims, poorly manufactured products, and economic harm.
One guardrail against unalloyed snake-oilsmanship is that dietary supplements can’t stray into the territory reserved for drugs. Under DSHEA and its parent statute, the Federal Food, Drug, and Cosmetic Act, a dietary supplement may not be marketed as intended to cure, mitigate, treat, diagnose, or prevent a disease, for that would cause it to be considered a drug, which does require premarket approval. The FDA has explained that general immunity claims like “supports the immune system” are not specific enough to be considered drug claims, but slightly more specific claims, such as “supports the body’s ability to resist infection,” are impermissible.
That is why it’s relatively rare to see claims like, “This product treats coronavirus!” Except when you do. In February, CSPI called out the erstwhile felon and current televangelist Jim Bakker for pushing a fake colloidal silver cure for coronavirus on his television show. Within two weeks, the FDA had issued a warning letter to “The Jim Bakker Show” ordering it to cease the sale of such unapproved products for COVID-19. The New York and Missouri Attorneys General followed up with legal actions, and Direct TV was told by its parent company AT&T to “carefully review” whether to take Bakker’s stations off the air.
Companies have long been walking the immunity tightrope—the thin line separating permissible immune function claims from fraudulent disease claims. And the moment a crisis raises its head, you can be sure that some unscrupulous dietary supplement makers will lower themselves to the challenge of producing unsubstantiated and often illegal claims. Amidst the COVID-19 pandemic, the FDA has issued warning letters to no fewer than 68 companies selling products (mostly supplements) making fraudulent claims to treat or prevent coronavirus infection, including several products making claims about COVID-19 immunity.

One special case is when manufacturers claim that their products belong to a recognized disease-treating drug class, such that, as the FDA explained in a 2002 Compliance Guide, “claiming membership in the product class constitutes a disease claim.” It singled out “antivirals” specifically.
Apparently, dozens of dietary supplement manufacturers didn’t get the memo. CSPI conducted a market scan on Amazon in late May and identified 46 products making specific antiviral claims like “effective against an enormous array of disease causing…virus[es],” “virus protection,” “and fend off certain viruses,” all clear violations of the Compliance Guide. So we wrote to the FDA and the Federal Trade Commission urging them to bring enforcement actions against these manufacturers. We also wrote to Amazon asking them to immediately delist these products.
But for every offending company caught in the FDA’s never-ending game of Whack-a-Mole, there are untold numbers of others that slip through the cracks or whose claims don’t quite rise to the level of a disease claim. Take this ad that popped up as Google became convinced that I was just another online supplement shopper. “Now, more than ever” is a reference to coronavirus if there ever were one, but some of these claims might just be vague enough to escape the FDA’s mallet.
After all, the chronically resource-deprived agency has to reserve its regulatory cudgel for the worst offenders. And the competition for that unenviable mantle is stiff. Still, we at CSPI are on the lookout on behalf of FDA and other agencies, so if you see a dietary supplement marketed to prevent or treat COVID-19, please write us at policy@cspinet.org.
This pandemic has turned into a field day for hucksters, carnival barkers, and purveyors of misinformation. It brings to mind H.G. Wells’s classic 1909 novel Tono-Bungay, in which a worthless elixir— characterized by the author as “nothing coated in advertisements”—is foisted upon an unsuspecting public as a nostrum for all that ails ye.
It doesn’t end well. The protagonist is left to contemplate a society that paid his uncle handsomely “for sitting in a room and scheming and telling it lies. For he created nothing, he invented nothing, he economized nothing. I cannot claim that a single one of the great businesses we organized added any real value to human life at all.
Tags
Topics
Contact Info: Contact us at cspinews[at]cspinet.org with questions, ideas, or suggested topics.

