Covid-19 vaccines: an interview with Dr. Anthony Fauci

POOL / REUTERS.
In mid-December, when the Pfizer vaccine started reaching the arms of healthcare workers (and the Moderna vaccine wasn’t far behind), Dr. Anthony Fauci spoke about the vaccine’s safety and effectiveness, and how to combat vaccine hesitancy, with CSPI President Dr. Peter Lurie.

Anthony Fauci has been director of the National Institute of Allergy and Infectious Diseases since 1984. He oversees research on HIV/AIDS, respiratory infections, diarrheal diseases, tuberculosis, malaria, Zika, and Ebola. He is also chief of the NIAID’s Laboratory of Immunoregulation, where his research has helped develop treatments for HIV, polyarteritis nodosa, and granulomatosis with polyangiitis. Fauci is a member of the White House Coronavirus Task Force and has been a voice for strong science throughout the pandemic.
A breakthrough
Lurie: Hi, Tony. Thanks so much for doing this. Let’s jump right in. How do you feel about the rapid development of these vaccines?
Fauci: Peter, I believe strongly that this is truly a historic scientific accomplishment. To go from the identification of a brand new pathogen in January 2020 to not only having a safe and highly effective vaccine, but one that’s already being administered to people, in about 11 months, this is beyond unprecedented. If this were a decade ago, or even five years ago, it would have taken several years at best.
Lurie: Would some people say that the speed was reckless?
Fauci: It has nothing to do with recklessness. It’s the application of extraordinary scientific advances, which have allowed us to do things in months that would have otherwise taken years.
In addition, it’s due to an extraordinary investment on the part of the government, to the tune of billions of dollars, to pre-purchase vaccines prior to the time that companies even demonstrated that they’re safe and effective, which means that you’re taking a financial risk.
If the vaccine works, you’ve saved many months. If the vaccine doesn’t work, you’ve lost a lot of money. The decision was made: Let’s gamble with the money to see if we can save time.
So when people express skepticism or concern about getting vaccinated because they feel it was done too quickly, it is important to explain that speed did not compromise safety or scientific integrity.
Lurie: Do you have any concerns about the FDA’s approval of the Pfizer vaccine?
Fauci: I was watching it like a hawk, Peter, and I can tell you that it was done in an entirely appropriate manner.
The data from the trials were given to a data and safety monitoring board, which is completely independent of the government, the drug company, and the FDA.
This board is a group of scientists, vaccinologists, immunologists, virologists, and statisticians who look at the data. And they determined that at a certain point, it was clear that the vaccine was safe and effective.
They then allowed the company to see the data, which the company analyzed, then submitted to the FDA to consider whether to approve the vaccine for an emergency use or for an outright licensure. The career scientists—not political appointees—at the FDA carefully scrutinized the data, and then the Vaccine and Related Biological Products Advisory Committee recommended that the FDA issue an Emergency Use Authorization.
Each of those steps occurred according to the way it should have occurred. And an EUA was granted, and now the vaccine is being distributed.
Skepticism is understandable, but what happened is a very clear, transparent, and independent process. We need to keep explaining that in a clear, concise way so that people understand.
Lurie: How was the vaccines’ effectiveness determined?
Fauci: By state-of-the-art, gold standard, randomized placebo-controlled trials. With the Pfizer vaccine, the trial included 44,000 people. With the Moderna vaccine, it was 30,000 people.
And at the end of the trials, it was shown that when you compare the number of clinically recognizable infections in the placebo versus the vaccine group, the vaccines were 94 to 95 percent effective, which in the world of vaccines is about as good as it gets.
Lurie: Did effectiveness vary by age, sex, race, or any other criteria?
Fauci: We monitor this carefully. About a third of the subjects in the Pfizer trial were aged 18 to 65 and healthy, a third were aged 18 to 65 with underlying conditions, and a third were older than 65.
And when you look at the data, it looks like the vaccine was effective across all of the groups—elderly, younger, and those with or without underlying conditions like obesity, hypertension, and diabetes, although you didn’t have enough people to do statistical tests on each of those groups.
What we don’t know until we give the vaccine to millions of people is how effective it’s going to be, for example, in people who are immunosuppressed, on cancer chemotherapy, or on other treatments that modify the immune system.
Vaccine vs. placebo: Who got Covid?
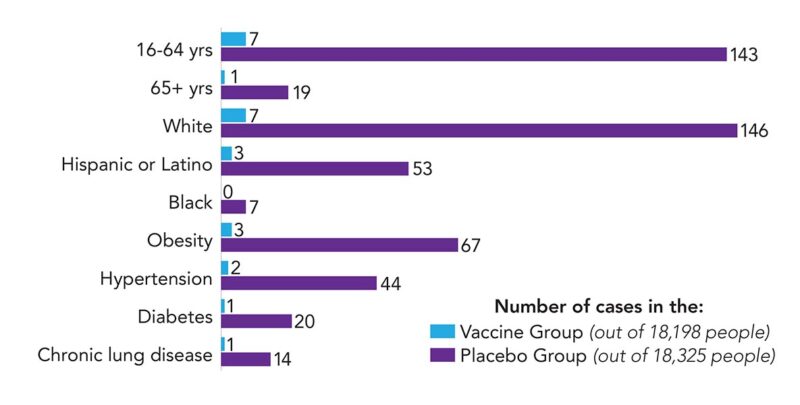
In the Pfizer trial, the vaccine cut illness from Covid regardless of age, race, or risk factors.
Safety
Lurie: What side effects have you seen?
Fauci: Let’s divide them into two buckets: what we call reactogenicity, which is what you get within 24 hours, sometimes within a couple of hours, and then the serious adverse events that could occur after weeks or beyond.
The Pfizer vaccine had reactogenicity that you might expect of a vaccine that elicits a strong immune response: pain in the arm, some swelling, some muscle aches, some fatigue, sometimes fever. They last generally between 24 and 36 hours, and they’re alleviated very nicely by something like Tylenol. We saw a significant amount of those reactions, which was not unexpected.
There were no serious adverse events that were red flags when the vaccine was rolled out in the UK in early December. However, two individuals there did have serious allergic reactions—anaphylactic-like reactions.
Lurie: Those reactions can be life-threatening because they can make you unable to breathe?
Fauci: Yes. Both had a significant history of severe allergic reactions. So Pfizer said, if you have a history of anaphylactic reactions to the ingredients in the vaccine, definitely don’t get vaccinated. And if you have a history of anaphylactic reactions to anything else, get vaccinated in the presence of someone or a facility that can handle a reaction.
If you have contact dermatitis or get mild allergic reactions to food or something else, you need not be concerned.
In the Pfizer trial, there were also a few cases of Bell’s palsy in the vaccine group. That’s a usually temporary weakness or paralysis of muscles in the face. However, the incidence was no greater than what you would see in the general population, regardless of vaccination. So the statisticians feel that Bell’s palsy is not associated with the vaccine.
The bottom line is that, apart from a couple of allergic reactions in people who had a strong history of allergic reactions, the safety profile looks very good.
Lurie: Could other adverse effects show up later?
Fauci: There is the possibility that you might have a serious consequence of the vaccine that you didn’t see in the first few weeks.
However, the FDA has looked at all of the adverse events that were reported in essentially all the vaccines they’ve ever approved. And they found that well over 90-plus percent occur within the first 30- to 40-odd days after the vaccination.
That’s why Pfizer had to wait 60 days from the time that half of the people received their last dose of the vaccine before it applied for an EUA. Historically, that should cover more than 90-plus percent of any adverse events that might occur.
So even though there’s one-year and two-year follow-up on this trial, you’ve already passed this 60-day period when most adverse events would be expected to appear.
Lurie: What might those long-term effects be?
Fauci: They’re mostly neurological—like a Guillain-Barré type syndrome, transverse myelitis, Bell’s palsy. It’s very, very rare that they occur that far out. But we’ve got to be alert that they are possible even though they’re extremely rare events.
Reluctance
Lurie: Why are some people afraid of the vaccine?
Fauci: It’s complicated. Some people are anti-vaxxers, and no matter what you tell them, they do not want to be pressured into getting a vaccine, because it’s an affront to their own personal decision making. They don’t want it, period.
But a far greater proportion of people might be hesitant or reluctant based on false information that they received, usually through social media.
We saw that very clearly with people reluctant to take measles vaccines because of the misinformation that it’s associated with autism, which is clearly not the case.
And once you show people the data, more often than not, you get them to reconsider. So you really need to be transparent with people to try to debunk the inaccurate information that they may have heard.
It’s a task. We’ve got to put the effort in to explain to people that in the history of preventive medicine, vaccines are overwhelmingly safe, and the benefits far outweigh the risks.
Lurie: Can you speak to concerns in the African-American community?
Fauci: Yes. There’s a mistrust on the part of the African-American community for any medical intervention, particularly one that’s being pushed by the government, which we are doing now because it’s our responsibility to end this outbreak.
When you look at the history of the relationship between the African-American population and the government’s health programs, the first thing that comes to mind is the disgraceful Tuskegee Study decades ago, where African-American men with syphilis were allowed to go untreated just to watch the progression of the pathogenesis of the infection.
That is terrible and should never have happened, and hopefully will never happen again, given the safety guidelines that we now have about clinical research.
But that’s something that sticks in the minds of people. So we’ve got to convince them that what we’re doing is for their own individual health, their family’s health, and, importantly, the health of society.
Lurie: What would you say to a family member of yours who is reluctant to take the vaccine?
Fauci: First of all, I wouldn’t denigrate them or make it like they don’t know what they’re talking about, because that’s the easiest way to shut somebody out.
Then I would try to get a feel for why they are reluctant. And then I’d go step by step, explaining why that concern might be understandable, but not justifiable.
Lurie: How does a decision to not get vaccinated affect others?
Fauci: It has profound implications, especially when you are dealing with a respiratory-borne illness like this one, where you don’t have any control over exposure or infectivity.
If enough people decide they don’t want to get vaccinated, we’ll never reach that protective umbrella of herd immunity, and this infection will always be a threat in society.
That’s why what someone else does might affect you, particularly if you’re a vulnerable person and you may not have a good immune response. You may need the protective umbrella of herd immunity to protect you.
Lurie: So we’re stronger together if we act together?
Fauci: No doubt.
Lurie: Do people who get vaccinated still need to wear a mask?
Fauci: Yes, they will, until the overwhelming majority of the population is vaccinated and there’s no virus circulating in society.
When you get some of the population vaccinated, but not as much as you need, and the level of infection goes way down, you don’t have to worry as much as we do now. You don’t have to avoid restaurants and theaters, for example.
But as long as there’s some infection in the community, you have to have some degree of wearing masks, avoiding crowds, avoiding getting close to people.
Lurie: Is that because people who get the vaccine could still infect others?
Fauci: Yes. The trials determined that the vaccines are 95 percent effective at preventing symptomatic disease. But they didn’t look at protecting against asymptomatic infection.
It’s possible that you could be protected against symptomatic disease but still have enough virus in your nose and throat to replicate. Hence, by definition, you are infected, and you might be able to infect others.
But you could also be infected but not have enough virus in your nose and throat to transmit it to anyone.
What’s more, we don’t know how long immunity will last.
Until we know the answers to those questions from other trials, even people who get the vaccines should wear masks and keep a distance from others.
Lurie: Will we ever go back to normal?
Fauci: I would like to say that we will have such good collective memory that we’ll be really careful about respiratory-borne illnesses, particularly in the winter. That is, people will avoid crowded places and might feel comfortable wearing a mask, as so many people in Asian countries do all winter. They do it as a courtesy, not to infect someone else, as well as to protect themselves.
Quite frankly, given my experience with how you forget things after they’re behind you, I think the everyday man and woman on the street probably will go back to the way things were before, with a little more attention to the fact that we could have another outbreak.
Lurie: Masks and distancing have an upside. I haven’t had a cold all year.
Fauci: You are like many people. I had the same experience. I always get two or three mild colds in the winter, and occasionally I might even get influenza, but I haven’t had a sniffle since last January.
In fact, that’s why we’re likely to have a mild flu season here. It’s already happened in Australia. Their winter ended in August, and they had the mildest flu season in recorded history.
And the reason is that people were doing things to prevent Covid-19. They were wearing masks and avoiding crowded situations, congregate settings, keeping a distance. And sure enough, the level of influenza almost disappeared.
Lurie: Thanks again for taking the time to talk to our readers, Tony.
Fauci: Take care, Peter.
This interview was condensed and edited for clarity.
For more information
- How the Covid mRNA vaccines work
- Covid vaccine myths & misinformation
- Centers for Disease Control and Prevention
To see a list of the ingredients in the vaccines, go to the Pfizer and Moderna websites.
Graph sources: FDA, N. Engl. J. Med. 2020. doi:10.1056/NEJMoa2034577.
Topics

